2020 Generic Drug & Biosimilars Access & Savings in the U.S. Report
Overview
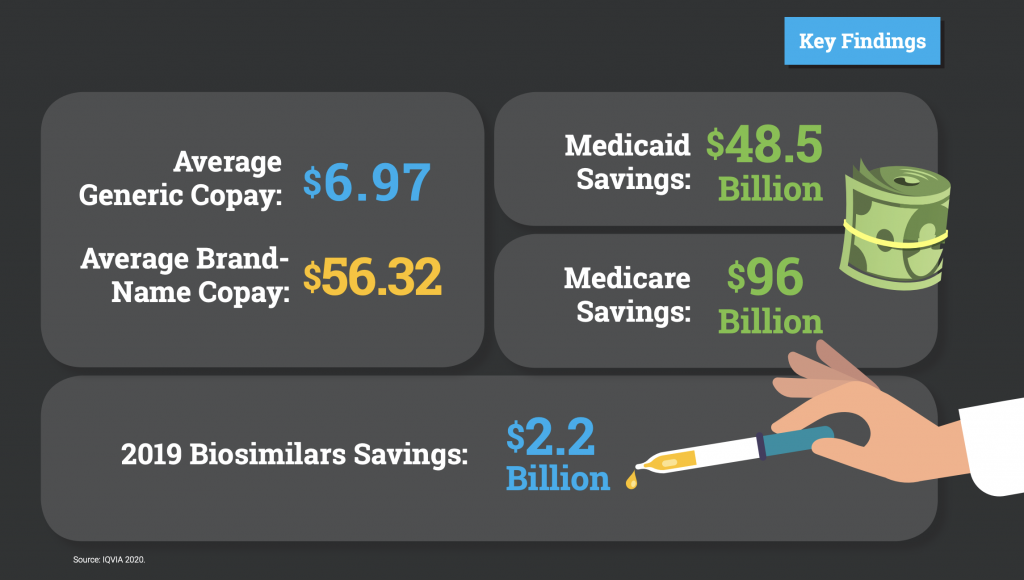
In 2019, the U.S. health care system saved $313 billion in 2019 from generics and biosimilar drugs, including $96 billion in Medicare savings and $48.5 billion in savings to Medicaid. At a time when access to reliable, affordable and high-quality prescription medication is more important than ever, biosimilar medicines saved patients and taxpayers $2.2 billion in 2019. The findings in this report reinforce the outsized importance of the generic and biosimilars industry in delivering lifesaving and health-managing medicines to patients.
Highlights
Below are some of the highlights from the 2020 Access & Savings report:
- In 2019, savings from use of biosimilar drugs reached $2.2 billion.
- Biosimilar medicines are projected to save $4.5 billion over the next 10 years.
- Since 2010, the FDA has approved 28 biosimilars but only 17 currently are available to patients. Greater use of these medicines, however, could generate even more savings.*
- Delayed launch of biosimilars due to patent thickets has cost the U.S. health care system an astounding $9.8 billion in lost savings since 2015.

Patient Case Study
Jeni Doerr, 35, has recently gone back to school to become a medical coder, and when she is not online learning she is outside enjoying everything California has to offer through hiking, rock climbing and mountain biking with her dog Sally.
Jeni takes the biosimilar Inflectra for her pancolitis. She had previously taken a brand biologic, but it didn’t do anything for her symptoms. “I have gained my life back through Inflectra, and I don’t have to worry about any expenses because it was covered. Had I known about biosimilars and Inflectra from the beginning I would have never tried anything else.”
Unfortunately, market obstacles, such as anti-competitive tactics, misguided government policies and rampant misinformation campaigns, risk patient access to lifesaving biosimilars and generics.
This year’s “Generic Drug Access & Savings in the U.S.” report released by AAM shares insight into the impact of biosimilars and generics to date and provides a critical look at how brands’ anti-competitive practices can negatively affect patients.
*Number of biosimilars approved through September, 2020.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.