This week, Avalere Health published new research that found 81% of health insurers are covering at least one of the two biosimilar products currently on the market in the United States. It’s no surprise that the biosimilar’s lower cost relative to the reference product was a key decision-making factor for nearly all payers. Like generic drugs, which saved the U.S. health care system $227 billion dollars in 2015, biosimilars have the potential to increase competition in the market, which will help lower the cost of biologic medicines and increase patient access to biopharmaceutical advances that increase the quality and length of their lives.
In addition, health insurers cited efficacy and safety of the biosimilar as important factors for coverage decisions. Biosimilars are subject to rigorous testing and review by the U.S. Food & Drug Administration (FDA) as well as monitoring after they are made available to patients in the U.S., giving confidence to patients and providers.
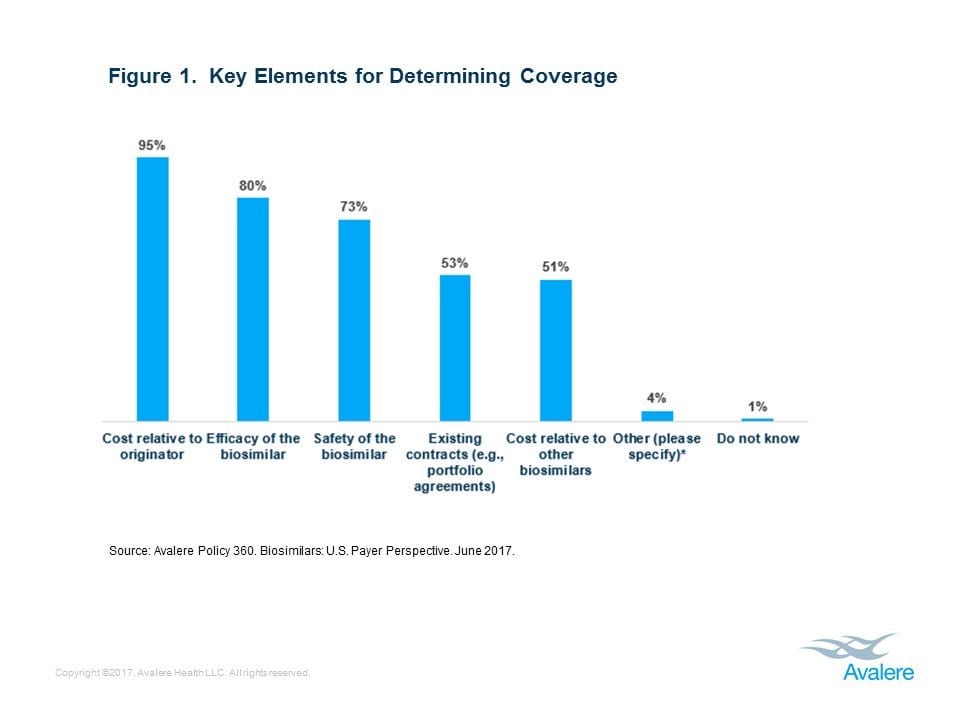
“Biosimilars were intended to increase competition among biologics and potentially reduce drug costs, but the biosimilars market has been slow to develop in the U.S.,” said Tom Kraus, senior vice president at Avalere. “The growth of the biosimilar market will be measured as policy, market, and intellectual property related challenges continue to be addressed.”
If you’re interested in learning more about biosimilars, join us at our annual conference, Leading on Biosimilars. Reserve your spot here: http://biosimilarscouncil.org/leading-on-biosimilars
The Biosimilars Council is now on Twitter, Facebook, and LinkedIn, follow us for the latest updates on biosimilars.
